Allergen immunotherapy involves administering one – or several – allergen extracts in order to induce the necessary tolerance to control the allergic response.
ALLERGEN EXTRACTS
Allergen extracts are heterogeneous mixtures of biological compounds obtained from natural allergen sources. When manufacturing these allergen extracts, raw material selection is crucial to the quality and final characteristics of the extracts.
Extraction and
purification of
allergen
extracts
PRICK TEST
The prick test is a sterile solution prepared to diagnose allergen hypersensitivity by puncturing the skin.
Prick test
manufacturing
process
SUBLINGUAL VACCINE
The sublingual allergen immunotherapy vaccine contains a glycerine solution of the extracted and purified allergen extract. It is prepared individually for each patient, according to the prescription of the specialist.
Sublingual vaccine
manufacture
DETERMINING THE BIOLOGICAL POTENCY
1.- Determining the in vivo biological potency (Biological standardisation)
According to the Nordic Guidelines, the biological activity of biologically standardised allergens is calculated through in vivo tests (skin prick tests) performed on a group of at least 20 allergic subjects sensitised to the allergen to be standardised.
The dose-response calculation is performed by relating the allergen concentration to the size of the papule induced by the positive control (histamine dihydrochlorohydrate). This test establishes the concentration of allergen that induces a papule equivalent in size to that produced by a histamine prick at 10 mg/mL. The median concentration of the allergen extract that causes a papule equivalent to that of histamine is the concentration that corresponds to 1,000TBU/mL for treatment, equivalent to 10,000 UB/mL. Thus, the biological reference standards are based on the total allergenic activity.
2.- Determining the in vitro biological potency
The aim of determining the biological activity is to correct qualitative and quantitative variations in each newly manufactured allergen extract, adjusting them to an appropriate final potency based on a reference extract. This way, we obtain high levels of efficacy and safety, as well as consistency among the new manufactured batches.
CHARACTERISATION
PROCESS
The characterisation process of allergenic extracts is based on a set of biochemical and immunochemical techniques, such as:
- Quantification of total proteins and determination of the protein profile.
- Quantification of the allergenic activity and allergen profile.
- Quantification of major allergens present in the allergenic extract.
The protein profile is analysed using the SDS PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) technique, which is based on the ability of proteins to move through the pores of a polyacrylamide gel when an electric field is applied. This movement will depend on the molecular size of the proteins, which allows proteins to be separated from the allergenic extract on the basis of their molecular size.
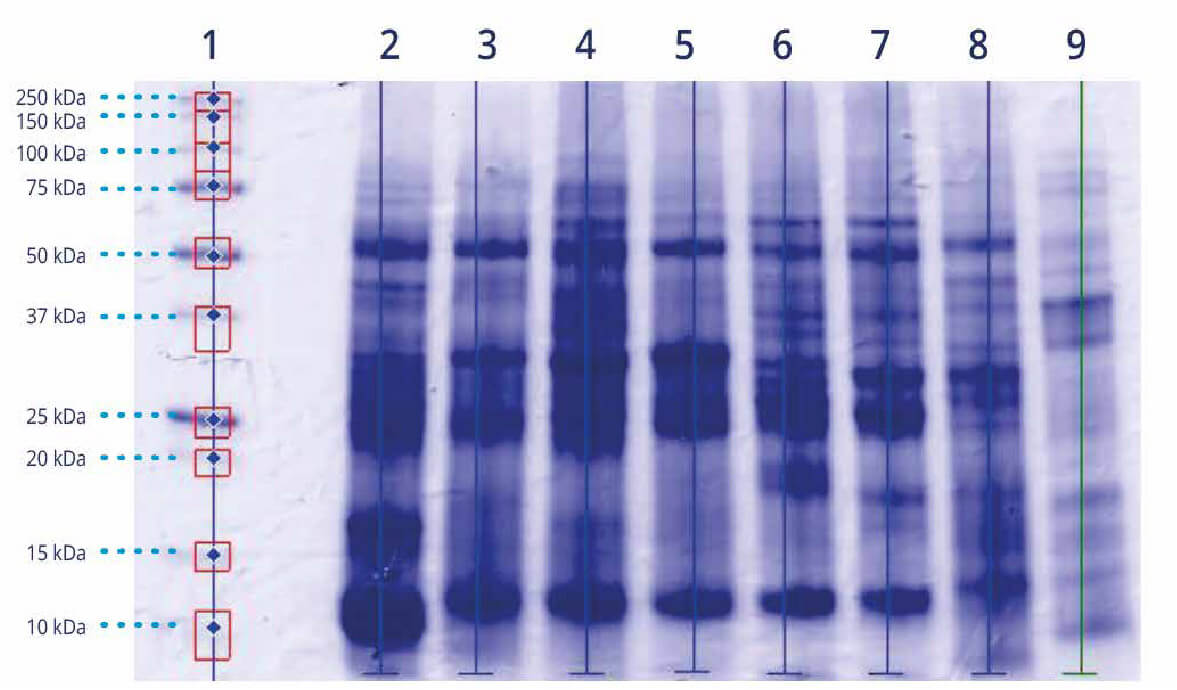
Protein profile of 7 grasses. MW (1), Phleum (2), Poa (3), Lolium (4), Dactylis (5), Hordeum (6), Secale (7), Triticum (8) + Olea (9) – Internal documentation-
